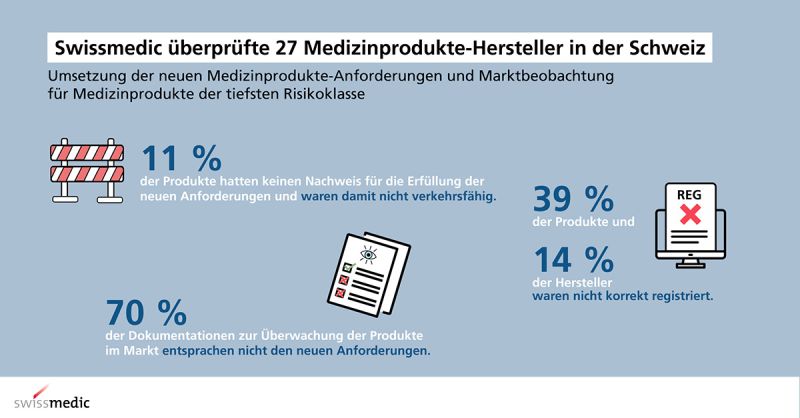
Swissmedic has performed inspections at class 1 manufacturers within Switzerland.
The result is impressive, but not surprising due to our experience as Authorised Representative in EU, Switzerland and UK.
During our onboarding process EUMEDIQ, we check not only completeness of Technical Documentation of our customer, we also do a plausibility check in order to estimate our business risk. Especially, class I manufacturers without notified body need to undergo an onboarding audit in order to assess the conformance of both, product (documentation) and quality management system. And we see that these class I manufacturers are often overwhelmed with the regulatory requirements.
For our business this means that class I manufacturers impose a higher risk and workload then manufacturers monitored by an accredited Notified Body.
Reach out to EUMEDIQ if you need to support in getting successful market access!